InspireMD Provides Business Update for the First Quarter of 2017
New Commercial Roll-Out of CGuard™ EPS on Schedule
84% Sequential Increase in Sales of CGuard™
Tel Aviv—May 9, 2017 – InspireMD, Inc. (NYSE MKT:NSPR) (NYSE MKT:NSPR.WS) (“InspireMD” or the “Company”), a leader in embolic prevention systems (EPS) / thrombus management technologies and neurovascular devices, today provided a business update, including an update on its new commercialization strategy. The Company also reported financial and operating results for the first quarter ended March 31, 2017.
James Barry, PhD, Chief Executive Officer of InspireMD, commented, “Earlier this year, we announced our transition away from a single distributor covering 18 European countries to a direct distribution model. In just a few short months, we have announced numerous distribution agreements covering markets across Europe, Asia and South America, fulfilling our commitment to relaunch both more broadly and more focused in Europe, as well as expanding our global footprint. We are extremely encouraged by the favorable response from our new partners and potential near-term future partners around the world. Not only have these distribution agreements expanded our geographic coverage, but in markets previously served by our former European distributor we are gaining deeper access into all four key clinical specialties that implant carotid stents.”
“While it has been a short time since we began signing these new distributors, the feedback has been very encouraging. Specifically, each of our new distributors is targeting the key opinion leaders (KOL) in its respective markets, and the response from many of these KOLs has been extremely positive. In fact, many of these KOLs who have not had access to the device until now, have become strong advocates for the CGuardTM EPS device and have begun to present at leading industry conferences such as ICCA Stroke 2017 in Russia and the Leipzig Interventional Course (LINC) 2017 in Germany, where a live case procedure performed by a leading German KOL was transmitted.”
“Despite the transition from our former distributor in the back half of the first quarter, we still managed to achieve 12% growth in year-over year sales of CGuard™ versus the same period last year. More importantly, we saw an 84% sequential increase in CGuardTM sales versus the fourth quarter of 2016, reflecting the beginning of our turnaround. Heading into the second quarter and balance of the year, we expect to generate both sequential and year-over-year growth in CGuard™ as we continue adding key hospitals and KOLs to our customer base around the world. As the device is put into the hands of new KOLs, it should, over time, lead to broader market adoption. As such, we expect more meaningful growth towards the end of this year and into 2018 as our primary customers shift from the small population of KOLs to the mainstream groups of vascular surgeons, interventional cardiologists, interventional radiologists and interventional neuroradiologists.”
“As these initiatives begin to take hold, we continue to maintain strict financial discipline and remain extremely confident in the outlook for the business.”
Financial Results
Revenue for the first quarter ended March 31, 2017 was $569,000 compared to $563,000 during the same period in 2016. The increase was primarily due to an increase in sales of CGuard™ EPS as we entered new regional markets despite the negative impact of the transition from our prior exclusive distribution partner for most of Europe. The transition to local distributors reflects an effort to broaden our sales efforts from only interventional neuroradiologists to include vascular surgeons, interventional cardiologists and interventional radiologists, as well. The increase in sales of CGuard™ EPS was partially offset by a decrease in MGuard™ Prime EPS associated with the trend of doctors increasingly using drug eluting stents (DES) rather than bare metal stents in STEMI patients. In the meantime, based on positive feedback from physicians, the Company is evaluating potential partners to combine MGuard™ Prime EPS with DES technology. Total operating expenses for the quarter ended March 31, 2017 were $2,478,000, an increase of 18.2% compared to $2,096,000 for the same period in 2016. This increase was primarily due to an increase in sales and marketing expenses (primarily to support the commercialization of CGuard™ EPS), as well as an increase in corporate related expenses. Net loss for the quarter ended March 31, 2017 totaled $2,559,000, or $0.81 per basic and diluted share, compared to a net loss of $2,252,000, or $7.00 per basic and diluted share, in the same period in 2016.
As of March 31, 2017, cash and cash equivalents were $8,572,000, compared to $7,516,000 as of December 31, 2016.
Conference Call
The Company will host a conference call on Wednesday, May 10 at 8:00 a.m. Eastern Time. The conference call will be available via telephone by dialing toll free 866-682-6100 for U.S. callers or +1 862-255-5401 for international callers, or on the Company’s Investor Relations section of the website:
https://www.inspiremd.com/en/investors/investor-relations/
A webcast will also be archived on the Company’s website and a telephone replay of the call will be available approximately one hour following the call, through midnight May 24, 2017, and can be accessed by dialing 877-481-4010 for U.S. callers or +1 919-882-2331 for international callers and entering conference ID: 10378.
About InspireMD, Inc.
InspireMD seeks to utilize its proprietary MicroNet™ technology to make its products the industry standard for embolic protection and to provide a superior solution to the key clinical issues of current stenting in patients with a high risk of distal embolization, no reflow and major adverse cardiac events.
InspireMD intends to pursue applications of this MicroNet™ technology in coronary, carotid (CGuard™), neurovascular, and peripheral artery procedures. InspireMD’s common stock is quoted on the NYSE MKT under the ticker symbol NSPR and certain warrants are quoted on the NYSE MKT under the ticker symbol NSPR.WS.
Forward-looking Statements
This press release contains “forward-looking statements.” Such statements may be preceded by the words “intends,” “may,” “will,” “plans,” “expects,” “anticipates,” “projects,” “predicts,” “estimates,” “aims,” “believes,” “hopes,” “potential” or similar words. Forward-looking statements are not guarantees of future performance, are based on certain assumptions and are subject to various known and unknown risks and uncertainties, many of which are beyond the Company’s control, and cannot be predicted or quantified and consequently, actual results may differ materially from those expressed or implied by such forward-looking statements. Such risks and uncertainties include, without limitation, risks and uncertainties associated with (i) market acceptance of our existing and new products, (ii) negative clinical trial results or lengthy product delays in key markets, (iii) an inability to secure regulatory approvals for the sale of our products, (iv) intense competition in the medical device industry from much larger, multinational companies, (v) product liability claims, (vi) product malfunctions, (vii) our limited manufacturing capabilities and reliance on subcontractors for assistance, (viii) insufficient or inadequate reimbursement by governmental and other third party payers for our products, (ix) our efforts to successfully obtain and maintain intellectual property protection covering our products, which may not be successful, (x) legislative or regulatory reform of the healthcare system in both the U.S. and foreign jurisdictions, (xi) our reliance on single suppliers for certain product components, (xii) the fact that we will need to raise additional capital to meet our business requirements in the future and that such capital raising may be costly, dilutive or difficult to obtain and (xiii) the fact that we conduct business in multiple foreign jurisdictions, exposing us to foreign currency exchange rate fluctuations, logistical and communications challenges, burdens and costs of compliance with foreign laws and political and economic instability in each jurisdiction. More detailed information about the Company and the risk factors that may affect the realization of forward looking statements is set forth in the Company’s filings with the Securities and Exchange Commission (SEC), including the Company’s Annual Report on Form 10-K and its Quarterly Reports on Form 10-Q. Investors and security holders are urged to read these documents free of charge on the SEC’s web site at http://www.sec.gov. The Company assumes no obligation to publicly update or revise its forward-looking statements as a result of new information, future events or otherwise.
Investor Contacts:
InspireMD, Inc.
Craig Shore
Chief Financial Officer
Phone: 1-888-776-6804 FREE
Email: craigs@inspiremd.com
Crescendo Communications, LLC
David Waldman
Phone: (212) 671-1021
Email: NSPR@crescendo-ir.com
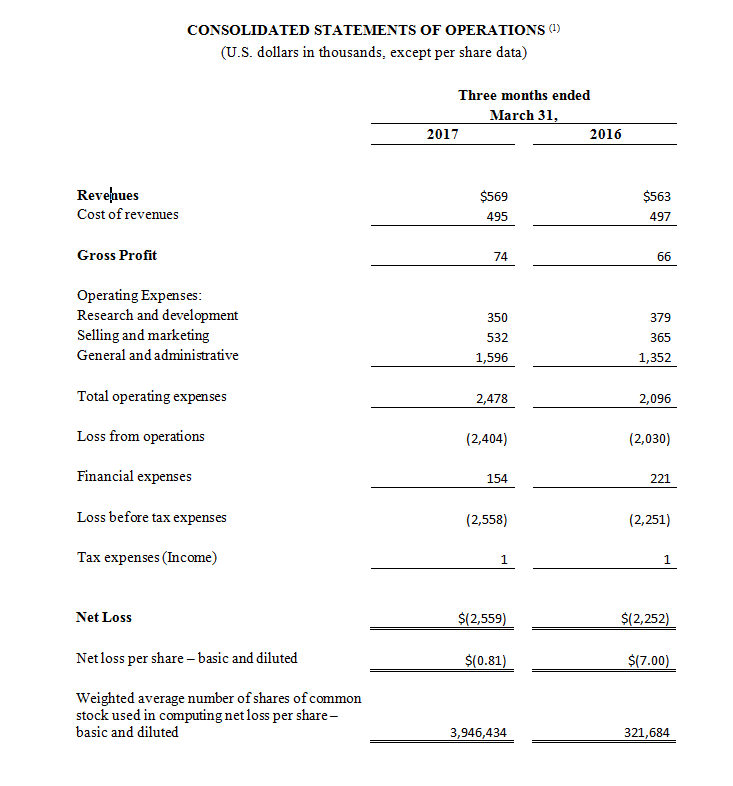
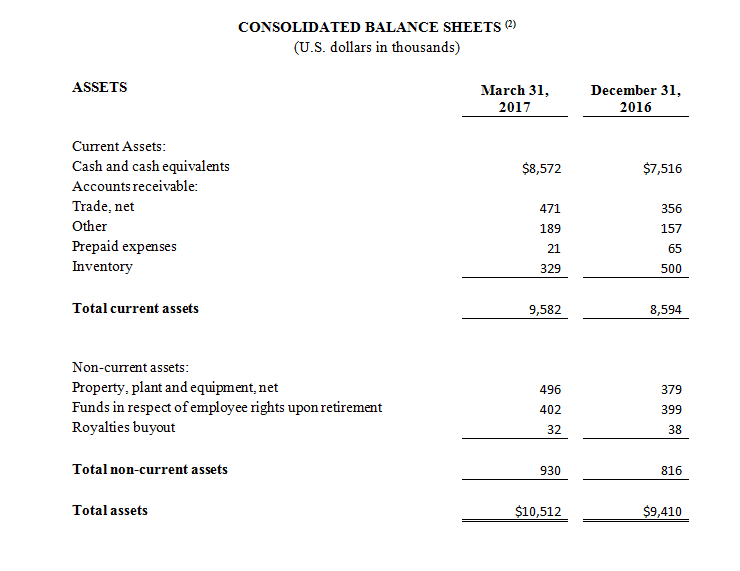
 (1) All 2017 financial information is derived from the Company’s 2017 unaudited financial statements, as disclosed in the Company’s Quarterly Report on Form 10-Q, filed with the Securities and Exchange Commission, all 2016 financial information is derived from the Company’s 2016 unaudited financial statements, as disclosed in the Company’s Quarterly Report on Form 10-Q, filed with the Securities and Exchange Commission.
(1) All 2017 financial information is derived from the Company’s 2017 unaudited financial statements, as disclosed in the Company’s Quarterly Report on Form 10-Q, filed with the Securities and Exchange Commission, all 2016 financial information is derived from the Company’s 2016 unaudited financial statements, as disclosed in the Company’s Quarterly Report on Form 10-Q, filed with the Securities and Exchange Commission.
(2) All March 31, 2017 financial information is derived from the Company’s 2017 unaudited financial statements, as disclosed in the Company’s Quarterly Report on Form 10-Q, filed with the Securities and Exchange Commission. All December 31, 2016 financial information is derived from the Company’s 2016 audited financial statements as disclosed in the Company’s Annual Report on Form 10-K, for the twelve months ended December 31, 2016 filed with the Securities and Exchange Commission.
