InspireMD Reports Financial Results for the First Quarter Ended March 31, 2016
BOSTON, MA – May 10, 2016 – InspireMD, Inc. (NYSE MKT: NSPR) (“InspireMD” or the “Company”), a leader in embolic prevention systems (EPS), neurovascular devices and thrombus management technologies, today announced its financial and operating results for the first quarter ended March 31, 2016.
During the first quarter, InspireMD announced initiatives to support ongoing commercial operations and development programs. Key activities include announced:
- Completed private placement and public offering;
- Strengthened Board of Directors with appointment of Isaac Blech as Vice Chairman; and
- Continued operational and financial alignment, with ongoing implementation of a comprehensive cash management program.
Sol Barer, Chairman of the Board of InspireMD, commented, “In line with our focused execution of our strategy, a key priority is our ongoing management transition plan with attention on our European commercial and development activities. These efforts were aided by our recent capital raise and addition to the board. We look for timely and continuous updates as we execute on our plans.”
Dr. Barer continued, “We remain pleased with consistent, positive feedback with our CGuardTM commercial activities, noting progress with our early efforts to drive market traction. We look forward to continued clinical presentations of CGuardTM patient evaluations at relevant medical industry conferences, including the investigator-led PARADIGM-101 at the Late-Breaking Clinical Trial session at EuroPCR on May 17th.”
Recent Operating Highlights:
Commercial
- Continued focus on expanding European commercial activities in new geographies.
- Successful CGuardTM commercial efforts across distributor network, particularly in select European regions, such as Italy.
Regulatory / Clinical / Product Development
CGuardTM evaluation on 101 consecutive all comer patients in PARADIGM-101 or PARADIGM-EXTEND study selected for Late-Breaking Clinical Trial presentation at EuroPCR conference from May 17-20, 2016 in Paris, France.
Financial
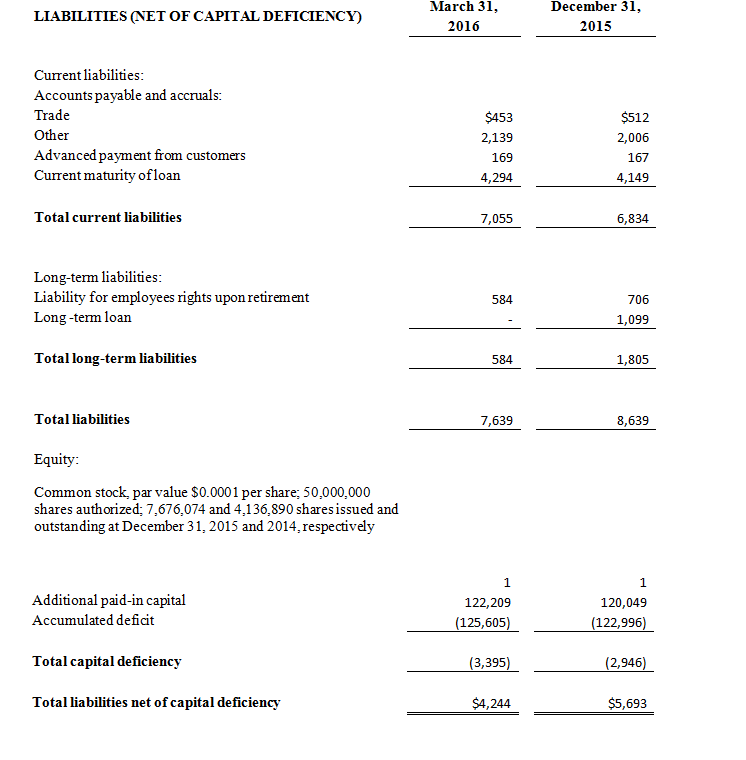
- Comprehensive and active cash management program, including a March 16th, 2016 pricing of an underwritten public offering of 1,900,000 shares of its common stock and warrants to purchase up to 950,000 shares of common stock and a concurrent private placement of 1,033,051shares of its common stock and warrants to purchase up to 516,526 shares of common stock to certain of the Company’s directors.
- Continued implementation of cost containment activities while supporting key development programs.
Quarter Ended March 31, 2016 Financial Results
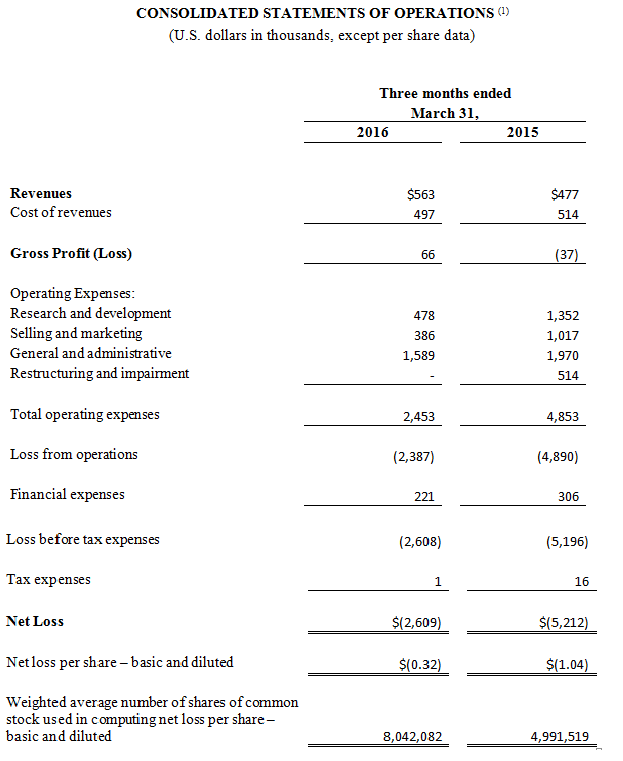
Revenue for the first quarter ended March 31, 2016 increased $0.1 million to $0.6 million compared to $0.5 million during the same period in 2015. The increase was predominantly driven by sales of $0.3 million of CGuard™ EPS, our carotid product. This increase, however, was partially offset by an expected decline in sales of MGuard™ Prime EPS associated with the trend of doctors increasingly using drug eluting stents rather than bare metal stents in STEMI.
The Company’s gross profit for the quarter ended March 31, 2016 was $66,000 compared to a gross loss of $37,000 for the same period in 2015. This increase in gross profit was largely attributable to the increase in product revenues and a decrease of write-offs of MGuard™ Prime EPS inventory offset by expenses related to the underutilization of our manufacturing resources.
Total operating expenses for the quarter ended March 31, 2016 were $2.5 million, a decrease of 49.5% compared to $4.9 million for the same period in 2015. This decrease was primarily due to a reduction of compensation related expenses, restructuring and impairment costs and other savings associated with our ongoing cost reduction plan.
The loss from operations for the quarter ended March 31, 2016 was $2.4 million, a decrease of 51.2% compared to a loss of $4.9 million for the same period in 2015.
Financial expenses for the quarter ended March 31, 2016 were $0.2 million, a decrease of 27.8% compared to the same period in 2015. This decrease was primarily due to a reduction in interest expense of our outstanding loan.
The net loss for the quarter ended March 31, 2016 totaled $2.6 million, or $0.32 per basic and diluted share, compared to a net loss of $5.2 million, or $1.04 per basic and diluted share, in the same period in 2015.
Non-GAAP net loss for the quarter ended March 31, 2016 was $1.9 million, or $0.23 per basic and diluted share, a decrease of 51.6% compared to a non-GAAP net loss of $3.8 million, or $0.77 per basic and diluted share, for the same period in 2015. The non-GAAP net loss for the quarter ended March 31, 2016 primarily excludes $0.7 million of share-based compensation. The non-GAAP net loss for the quarter ended March 31, 2015 primarily excludes $1.0 million of share-based compensation and $0.3 million of expense related to an impairment of a royalties buyout asset.
Cash and Cash Equivalents
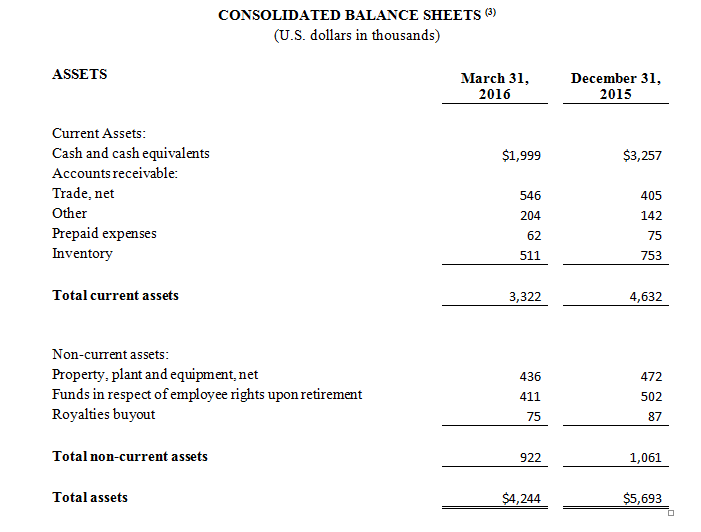
As of March 31, 2016, cash and cash equivalents were $2.0 million, compared to $3.3 million as of December 31, 2015.
No Conference Call Scheduled
The Company is not hosting a conference call to discuss first quarter March 31, 2016 results. Additional updates will be provided as they become available.
About PARADIGM
PARADIGM is an investigator-initiated Prospective evaluation of All-comer peRcutaneous cArotiD revascularization In symptomatic and increased-risk asymptomatic carotid artery stenosis, using CGuard™ Mesh-covered embolic prevention stent system. At EuroPCR 2015, Dr. Musialek summarized the results of his PARADIGM evaluation of 71 CGuardTM procedures in unselected all-comer patients: 1) stent system success and procedure success rate of 100%; 2) periprocedural complications of 0%, and remained at 0% at 30 days; and 3) no MACNE occurred periprocedurally or at 30 days, by operator-independent neurologist and non-invasive cardiologist evaluation.
About InspireMD, Inc.
InspireMD seeks to utilize its proprietary MGuard™ with MicroNet™TM technology to make its products the industry standard for embolic protection and to provide a superior solution to the key clinical issues of current stenting in patients with a high risk of distal embolization, no reflow and major adverse cardiac events.
InspireMD intends to pursue applications of this MicroNet™ technology in coronary, carotid (CGuardTM), neurovascular, and peripheral artery procedures. InspireMD’s common stock is quoted on the NYSE MKT under the ticker symbol NSPR.
Forward-looking Statements
This press release contains “forward-looking statements.” Such statements may be preceded by the words “intends,” “may,” “will,” “plans,” “expects,” “anticipates,” “projects,” “predicts,” “estimates,” “aims,” “believes,” “hopes,” “potential” or similar words. Forward-looking statements are not guarantees of future performance, are based on certain assumptions and are subject to various known and unknown risks and uncertainties, many of which are beyond the Company’s control, and cannot be predicted or quantified and consequently, actual results may differ materially from those expressed or implied by such forward-looking statements. Such risks and uncertainties include, without limitation, risks and uncertainties associated with (i) market acceptance of our existing and new products, (ii) negative clinical trial results or lengthy product delays in key markets, (iii) an inability to secure regulatory approvals for the sale of our products, (iv) intense competition in the medical device industry from much larger, multinational companies, (v) product liability claims, (vi) product malfunctions, (vii) our limited manufacturing capabilities and reliance on subcontractors for assistance, (viii) insufficient or inadequate reimbursement by governmental and other third party payers for our products, (ix) our efforts to successfully obtain and maintain intellectual property protection covering our products, which may not be successful, (x) legislative or regulatory reform of the healthcare system in both the U.S. and foreign jurisdictions, (xi) our reliance on single suppliers for certain product components, (xii) the fact that we will need to raise additional capital to meet our business requirements in the future and that such capital raising may be costly, dilutive or difficult to obtain and (xiii) the fact that we conduct business in multiple foreign jurisdictions, exposing us to foreign currency exchange rate fluctuations, logistical and communications challenges, burdens and costs of compliance with foreign laws and political and economic instability in each jurisdiction. More detailed information about the Company and the risk factors that may affect the realization of forward looking statements is set forth in the Company’s filings with the Securities and Exchange Commission (SEC), including the Company’s Annual Report on Form 10-K and its Quarterly Reports on Form 10-Q. Investors and security holders are urged to read these documents free of charge on the SEC’s web site at http://www.sec.gov. The Company assumes no obligation to publicly update or revise its forward-looking statements as a result of new information, future events or otherwise.
Investor Contacts:
InspireMD, Inc.
Craig Shore
Chief Financial Officer
Phone: 1-888-776-6804 FREE
Email: craigs@inspiremd.com
PCG Advisory
Vivian Cervantes
Investor Relations
Phone: (212) 554-5482

(1) All 2016 financial information is derived from the Company’s 2016 unaudited financial statements, as disclosed in the Company’s Quarterly Report on Form 10-Q, filed with the Securities and Exchange Commission, all 2015 financial information is derived from the Company’s 2015 unaudited financial statements, as disclosed in the Company’s Quarterly Report on Form 10-Q, filed with the Securities and Exchange Commission.
(2) Our non-GAAP net loss is presented as management uses this supplemental non-GAAP financial measure to evaluate performance period over period, analyze the underlying trends in our business, and establish operational goals and forecasts that are used in allocating resources. We believe by presenting this additional measurement, we are providing investors with greater transparency to the information used by our management for our financial and operational decision-making, as well as allowing investors to see our results “through the eyes” of management. We further believe that providing this information assists our investors in understanding our operating performance and the methodology used by management to evaluate and measure such performance.
(3) All March 31, 2016 financial information is derived from the Company’s 2016 unaudited financial statements, as disclosed in the Company’s Quarterly Report on Form 10-Q, filed with the Securities and Exchange Commission. All December 31, 2015 financial information is derived from the Company’s 2015 audited financial statements as disclosed in the Company’s Annual Report on Form 10-K, for the twelve months ended December 31, 2015 filed with the Securities and Exchange Commission.
