
Perspectives from the IDE Trial PI and a Passionate Carotid Stenter

OK great thank you very much Marvin. A quick check can everybody see my slides OK? OK, well first of all it’s an honor to be with this panel, with you Marvin with the team, and with potential investors to share a passionate carotid stenter’s perspective on this technology and also from the IDE trial going on in the United States.

Here are my disclosures again, importantly I am the national PI for the trial in a compensated role and I’ve been the PI for other carotid stent trials as well, I promise this is from the heart not from the wallet my discussions here.
So it’s important background, as Marvin mentioned but now for the first time we see a definite trend for towards carotid stenting away from endarterectomy, even from vascular surgeons with TCAR and carotid stenting. There is a lot of carotid disease out there and as everyone knows patients are terrified of themselves having a stroke. Carotid stenting, as mentioned, has had some issues with minor periprocedural strokes. An ideal carotid stent is needed to reduce these types of strokes, to conform to the anatomies of the vessels that we treat, and to be usable whichever way we choose to get to the carotid, whether it’s TCAR, transfemoral, or transradial, etc.
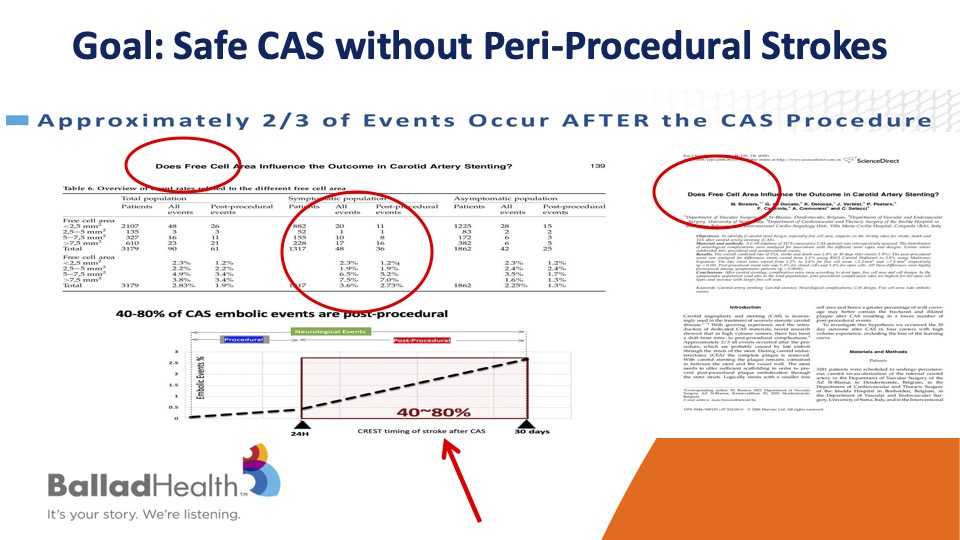
So our goal of course is carotid stenting performed safely without any periprocedural stroke.
It’s very important to realize that the majority of strokes after carotid stenting don’t occur during the procedure but rather in that short time interval after it, OK? And what that means in various studies is that the more the cell is open, in other words the biggest size in the stent pores, the more likely that plaque is able to get through the stent later, and then go upstream even though you had a nice result.
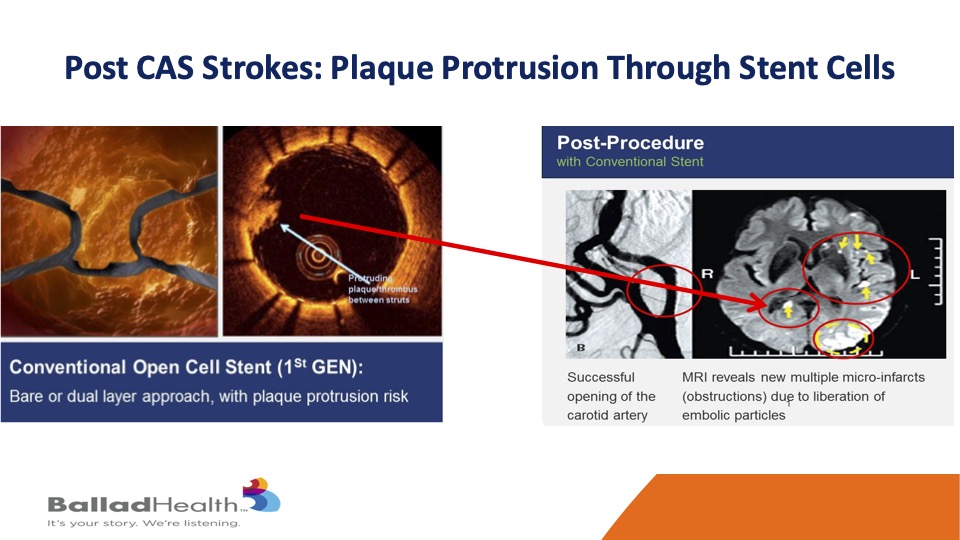
Marvin showed this is a standard stent over here and you see the coverage is not so great, and even though the stent looks good for most of it, there’s that little plaque protrusion at the arrow so even though the angiogram looks good, the brain does not look good if some of that material extrudes, gets through the stent after the procedure and gets to the brain.
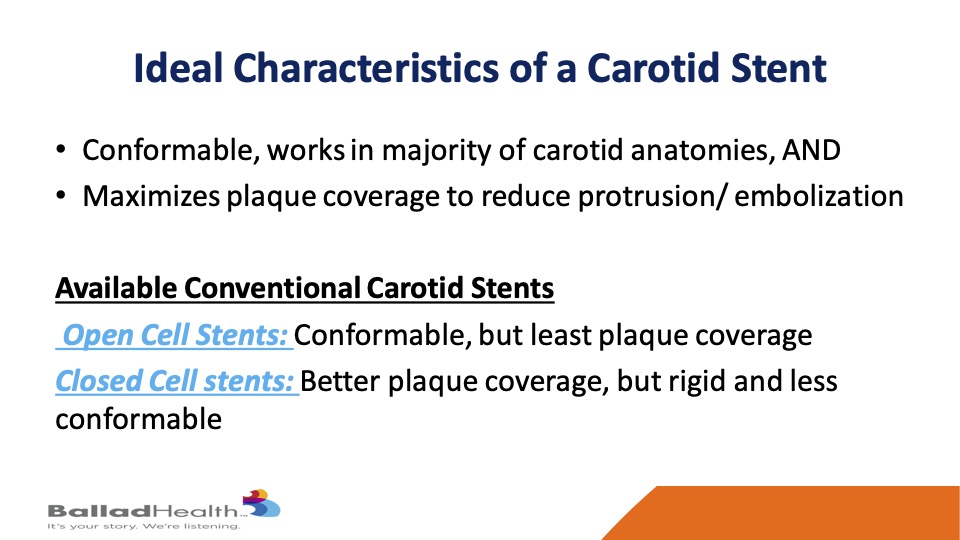
Now what would be an ideal carotid stent? It would conform to the majority of the vessels that we treat and yet maximize plaque coverage to reduce particles get up to the brain on later basis. Well, the available conventional stents that we’ve had for years are either open cells, the great thing is they’re conformable, they’ll go around bends, but they have the least plaque coverage. Then there are closed cell stents, the coverage is better but these are more rigid tubes and less conformable and as you’ll see it’s still not ideal plaque coverage.

The CGuard micromesh carotid stent maybe that ideal stent, covering this MicroMesh layer that we look at on a conformable stent.

It may combine the best of two worlds- It’s extremely conformable, as well as anything that’s been out there, through that proprietary SmartFit technology, and yet has by far the best plaque coverage through the micro mesh layer with the smallest stent cell size that have been seen to this day.
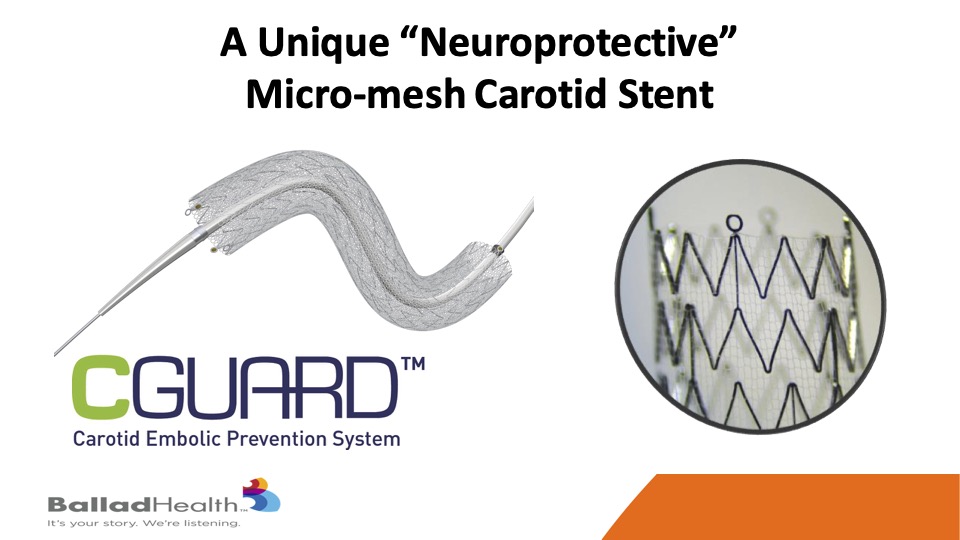
So here you see this conventional stent and the metal struts covered with this much smaller micro mesh layer that’s on the outside of stent, which is important, on this conformable design with increased coverage.

You’ve seen this, this is just a conventional stent, and sewn on the outside is this micro mesh layer.
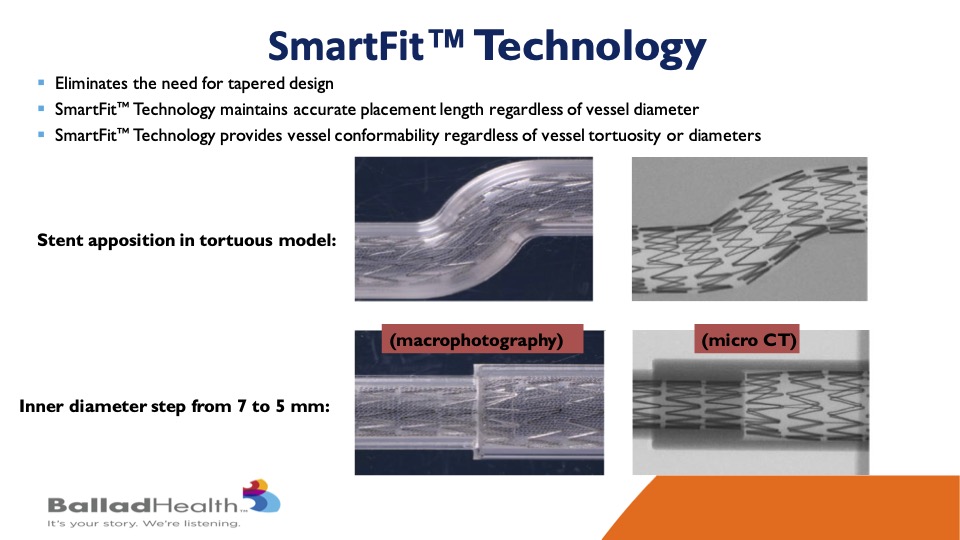
You’ve seen the Smart Fit technology, but what I will say is that now you don’t have to have her taper with a 7 millimeter one side 10 millimeter at the other and then a twist of things in between. You can have one stent that itself will conform to both ends as you said reducing the inventory that we need as physicians and Sean Lyden and others need as supply chain directors, this is a good thing.
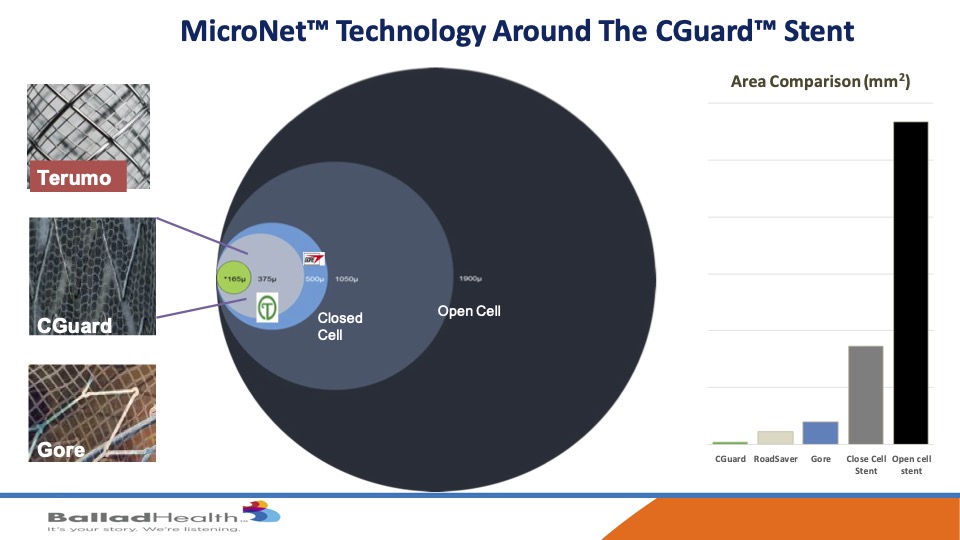
Despite this comfortability, it has unbelievable plaque coverage. If you look here the open cell this huge circle, closed cell is best we’ve had look, at that size the other two micro mesh carotid stents are here and then in green is CGuard, and if you look at on a graph over here you can see how much smaller the pore size is the opening here to let plaque not get through is what we’re trying to do.

So going back to our initial picture you start with this plaque protrusion through this metal layer and replace it with the same picture without plaque protrusion because it’s extra insulating layer or protective layer.

Now there are two other micromesh stents, in Chris’s opinion, I’ve been in all three of the trials, I think this stent has advantages. it has an increased conformability with kind of an auto tapering OK. It’s an exact standard 40 millimeter stent is a 40 millimeter stent as opposed to others that greatly elongate where you don’t need it. The mesh sizes are smaller, the pore size are smaller and it’s on the outside of the stent so it’s not infolding inside this tent and there’s no coating on this. In the data, long term data has a lot less re-stenosis or occlusion compared to the others and durable than. It’s easier to size and deliver and there’s a large matrix of stent sizes available and no supply chain issues.

We’ve looked at this, but it is important again, this is a slide Marvin showed, but as a doc, hey I can show you great animations, but we want to see data and the bottom line is in 27,000 implants and 1600 in rigorous clinical trials you can see durable results up to five years and again this is where we see the advantages against the other stents.
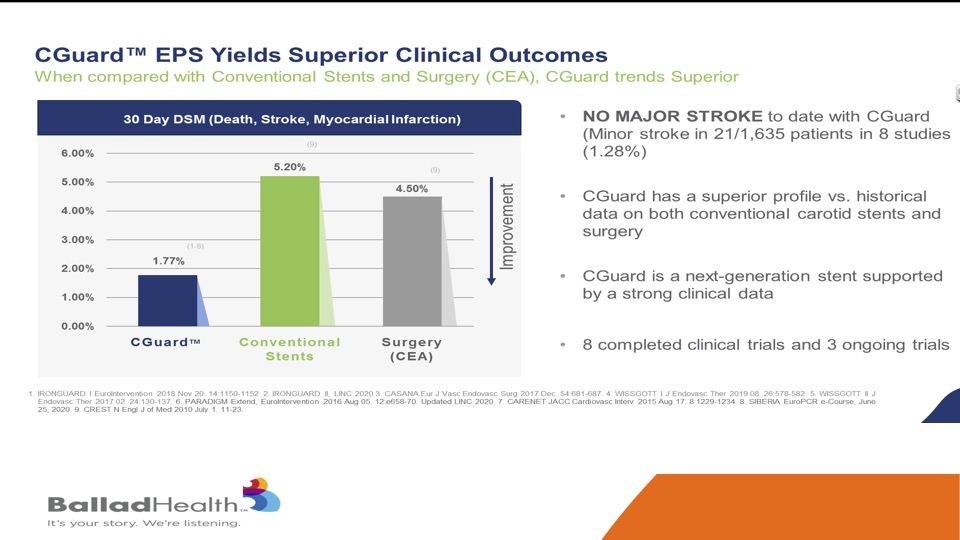
Again, you’ve seen this slide as well, this is not a direct comparison, but this is the conventional stents and surgery in the CREST randomized trial in the New England Journal, so this is very carefully adjudicated data and you can see the CGuard performed very favorably and no major strokes seen with this protection against plaque protrusion.

A brief word about the CGuardiands US IDE pivotal trial, this is for US approval, for CMS and others- we’ve done this very carefully. Christina Brennan and others and Marvin, they’ve done a great job with this. We require either the NAV 6 distal protection, the best in class for distal filter protection, or the MoMa proximal protection those are the only two you are allowed, we know these are the best protection systems. The sites and operators are very carefully selected, cases must be approved by an experienced screening committee to get into the trial, so we’re not letting bad cases in the trial. The protocol has been carefully prepared and undergoes revisions on a regular basis from anything that we learn that wasn’t perfect. We review the cases after they’re done, and so what you have is what I think is an excellent stent combined with an excellent study with experienced sites, operators, mandated procedural techniques and the right technology and carefully selected and reviewed cases.
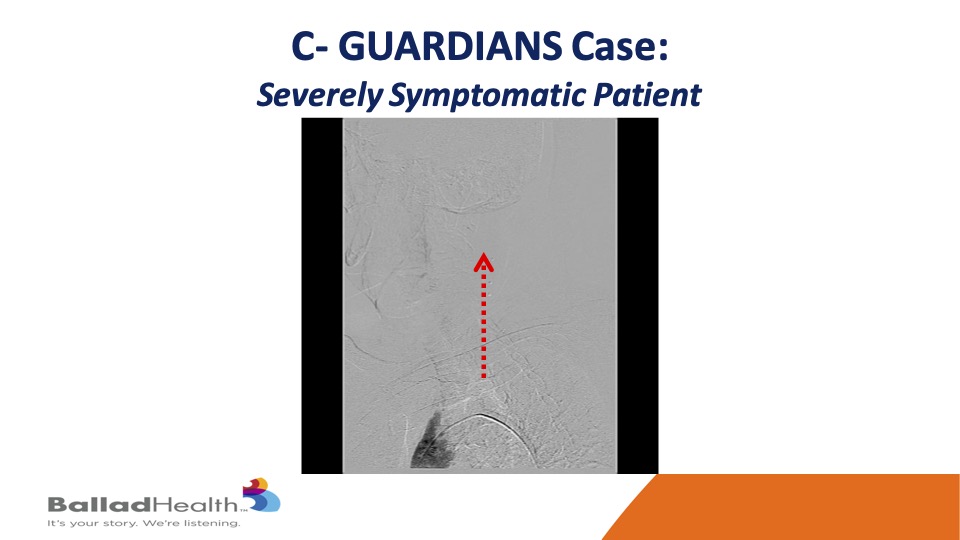
Here’s one of our cases in the trial- you can see the aortic arch here and very slow flow in that red dotted arrow and the left carotid system.
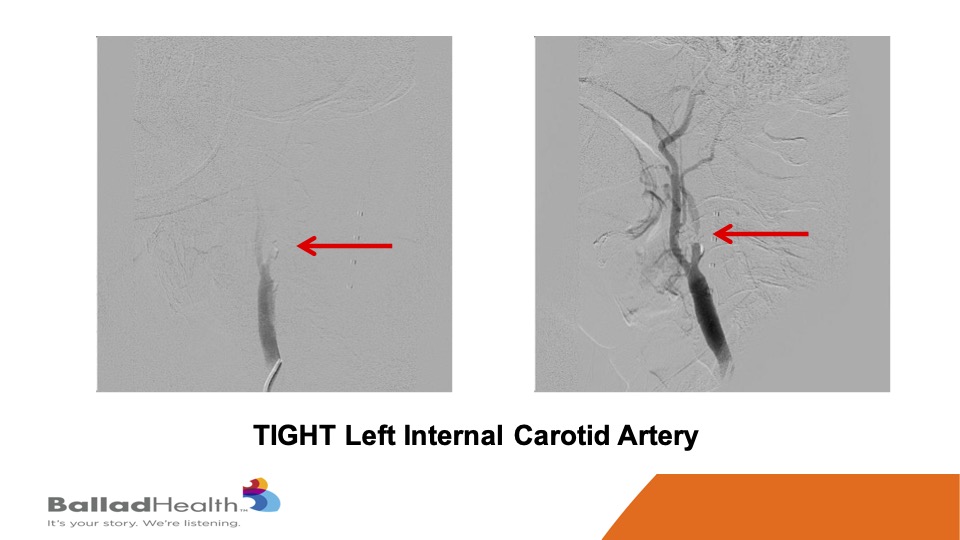
This is a severely symptomatic patient, you can see how tight at the red area of the carotid is you really want protection for this kind of person who’s already thrown a little pieces to her brain.
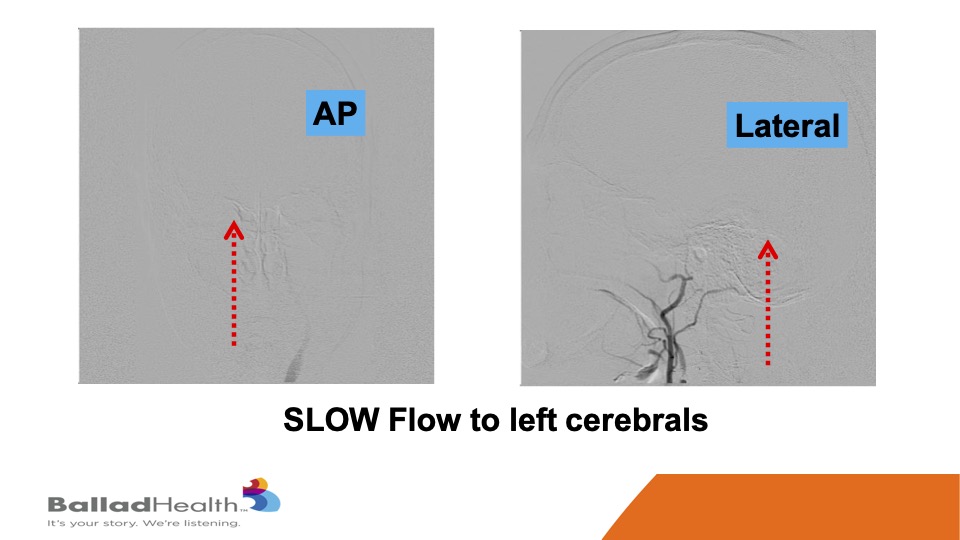
Look how slow the flow is getting to the brain and the dotted arrows that’s at baseline.
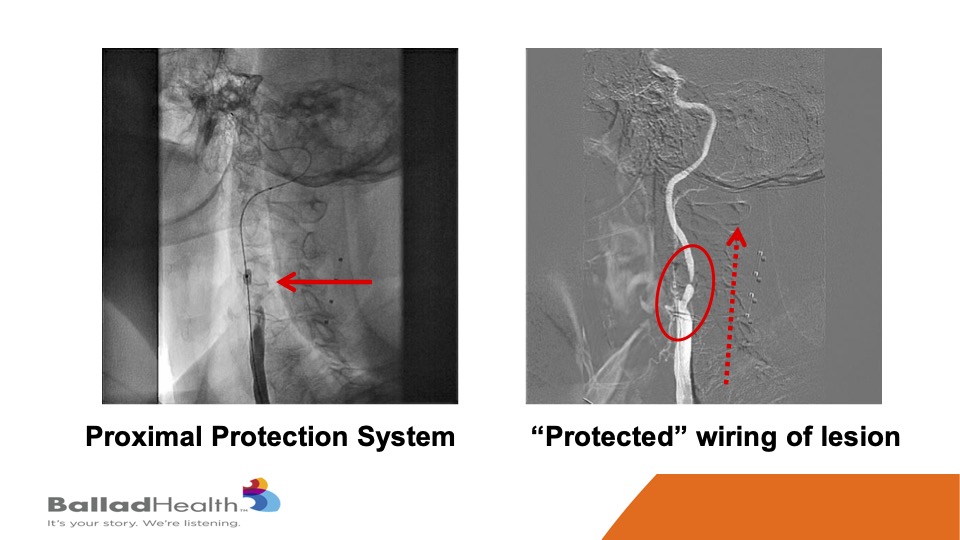
So, here’s the proximal protection system. This is a balloon in the external carotid, we’re going to shut the blood supply, and in the red circle you’ll see me steering a 0.014 inch wire in a protected fashion OK. With the protection we do a balloon, and then in the red dotted arrow this is a CGuard being released, and it comes out into the carotid artery.
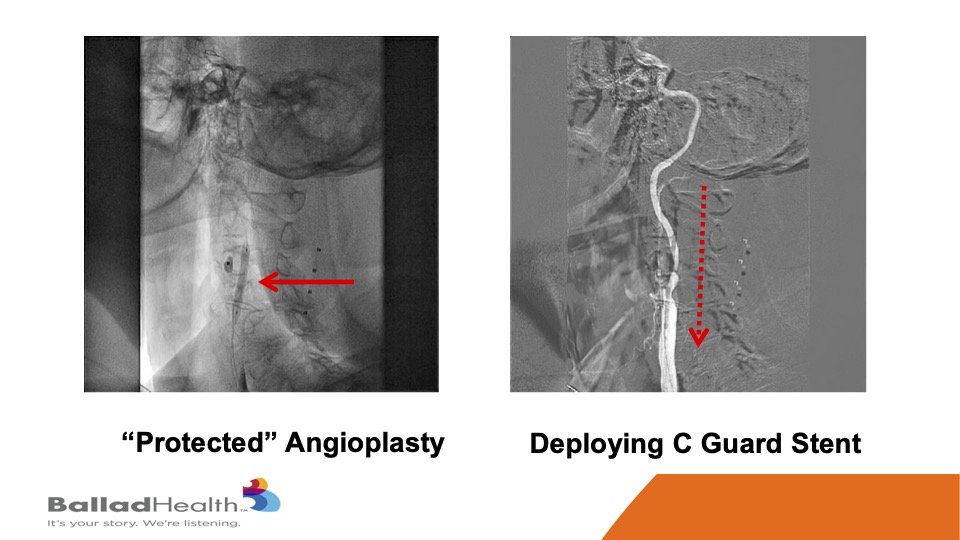
This is that stent, and then we inflate a balloon within the stent.
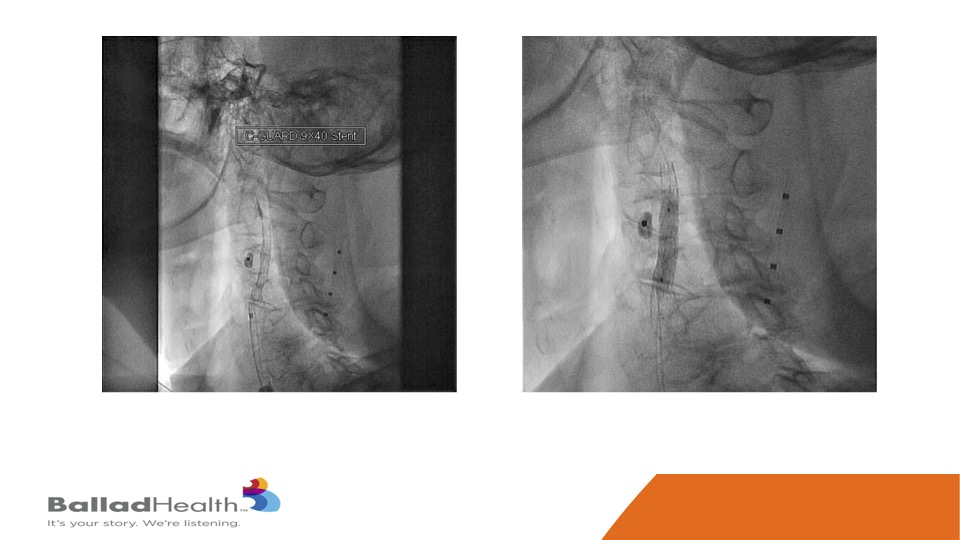
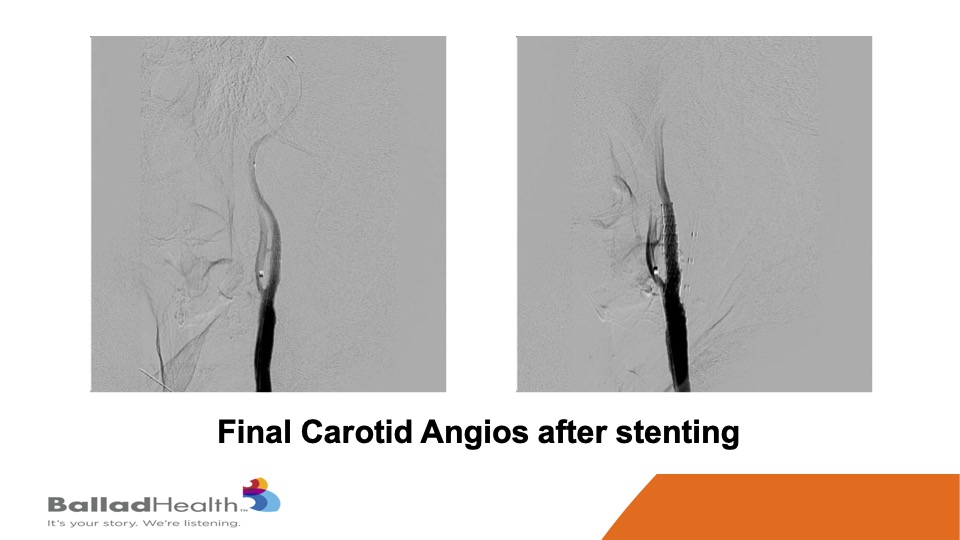
Here’s the final nice result, with the protection of the micro mesh, to prevent any of this from getting up to the brain now or later.
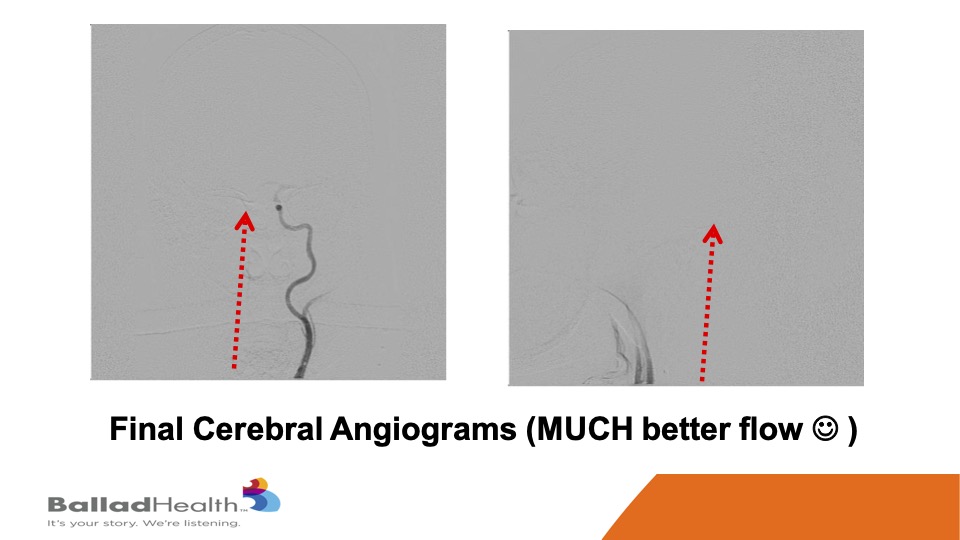
Look how brisk the flow is compared to what I showed you before. You know this patient right after this was asking me immediately for calculus book which we had to deliver to the bedside. I may be exaggerating slightly, but the flow was a lot better.

So if I was an investor, I’m not, I’m a plumber, so this is up to you guys but if I was an investor, why would I consider investing here? I think it’s an awesome stent, I believe it, and it’s got a lot of data and experience already with more accumulating. The company is a nice company. When you have savvy leadership and yet they are user-friendly, you all know and we know it as docs, it’s nice to have that combination, and user friendliness I believe it’s there. There’s a potential that CMS is currently reviewing the national coverage decision so that we may even expand coverage for carotid stenting. That comes, we have a predictable IDE trial that is ongoing now, predictable is an air quotes, but you know we’ve designed it to be a win for the stent because it’s a good stent and done correctly in a good trial. Again, there’s already an increasing trend toward carotid stenting even amongst the vascular surgeons, couple that with increasing reimbursement options, it may be a very good time. And again this stent is going to be designed in such a way, and again these are the forward thinking statements, so it could be compatible with TCAR which the vascular surgeons are embracing, or from transfemoral and there are other new indications and devices being tested in their technology pipeline.

So in conclusion, InspireMD has a very unique stent and a lot of suggestions that it will be the best in class carotid stent. There’s already excellent experience and data long term for this. In the US, the pivotal IDE trial is enrolling well, with very favorable results to date, and expected to continue with favorable results. Increasing trend towards carotid stenting and potential increases in reimbursement, in my opinion, make this a unique time and opportunity for mutually beneficial partnerships amongst all of us.




