InspireMD Reports Third Quarter 2022 Financial Results and Provides Business Update
– Generated 38.8% growth in CGuard™ revenue year-over-year –
– Continued enrollment in the C-Guardian US IDE trial, with 24 sites currently enrolling patients; on track to complete enrollment by approximately end of Q1 2023 –
– Announced strategic partnership with NAMSA, a med tech contract research organization (CRO), to accelerate new medical device development and commercialization –
—
Management to Host Investor Conference Call Today, November 8th, at 8:30am ET
—
Tel Aviv, Israel— November 8, 2022 – InspireMD, Inc. (Nasdaq: NSPR), developer of the CGuard™ Embolic Prevention System (EPS) for the treatment of carotid artery disease (CAD) and prevention of stroke today announced financial and operating results for the third quarter ended September 30, 2022.
Third Quarter 2022 and Recent Highlights:
- CGuard revenues for the third quarter 2022 were $1,430,836, a 38.8 % increase over the same period in 2021, on 2,624 stent systems sold, as compared to 1,709 stents sold in the same period in 2021.
- Continued enrollment in the C-Guardian Investigational Device Exemption (IDE) Clinical Trial. The company currently has 24 trial sites enrolling patients (19 US, 5 EU) and anticipates adding two more US sites by the end of the year. The company anticipates completing enrollment by approximately end of Q1 2023, consistent with prior guidance.
- Professor Piotr Musialek presented at the 2022 Transcatheter Cardiovascular Therapeutics (TCT) Conference long term patient results from the ongoing CGuard Optima study establishing a new baseline of carotid stent performance.
- Announced strategic outsourcing partnership with NAMSA, a med tech contract research organization (CRO), to accelerate new medical device development and commercialization.
Marvin Slosman, CEO of InspireMD, commented: “During the third quarter, we continued to gain share in our key European markets, contributing to nearly 40% CGuard revenue growth over the prior year period. We continue to work with our Notified Body to secure our CE Mark certification under the MDR, which currently expires November 12, while preparing our customers and distributors with sufficient inventory to mitigate as best as possible any potential delay in the recertification process.
“At the same time, our U.S. IDE trial now has 24 sites enrolling patients. We continue to anticipate having the trial fully enrolled by approximately end of Q1 of next year, a critical step forward in our goal to gain eventual marketing approval in the U.S.”
“We believe we have set the stage for a catalyst-rich 2023, driven by continued share gains in our established markets, ongoing progress with our U.S. IDE trial, conversion of existing endovascular CAD procedures to CGuard from other stent systems, and the introduction of two new delivery systems, our SwitchGuard Trans Carotid (TCAR) , and CGuard Prime Transfemoral (TFEM) platforms which will allow us to continue to address the comprehensive needs for all vascular specialist treating Carotid disease and stroke, including vascular surgeons who continue to treat a significant percentage of Carotid patients,” Mr. Slosman concluded.
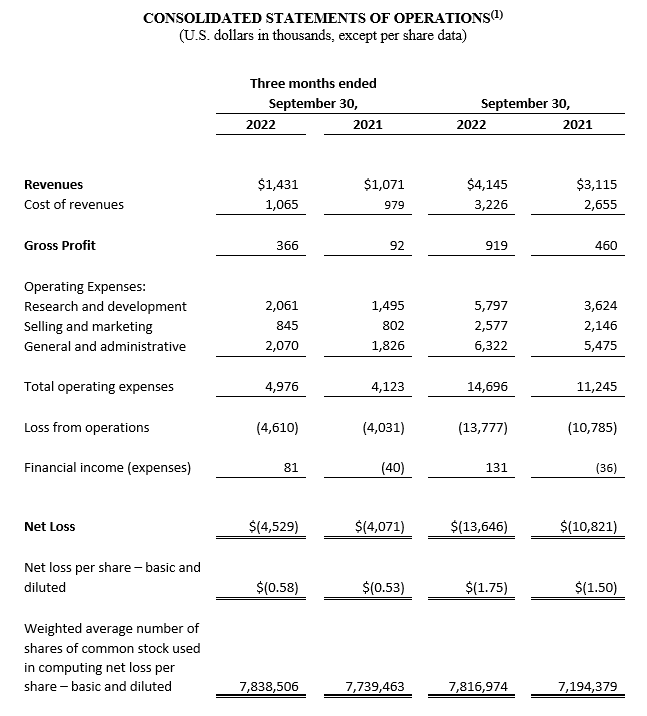
Financial Results for the Third Quarter ended September 30, 2022
For the third quarter of 2022, total revenue increased 33.6%, to $1,431,000, from $1,071,000 during the third quarter of 2021. This increase was predominantly driven by a 38.8% increase in sales of CGuard EPS, to $1,431,000 in the third quarter of 2022 from $1,031,000 in the same period one year ago. This sales increase was due to growth in existing markets as well as US sales related to stents used in the C-Guardian U.S. Food and Drug Administration (FDA) clinical trial.
Gross profit for the third quarter of 2022 increased by $274,000, or 297.8%, to $366,000, compared to a gross profit of $92,000 for the third quarter of 2021. This increase resulted from higher revenue and a reduction in write offs of $64,000, training expenses of $64,000 and miscellaneous expenses of $51,000. Gross margin (gross profits as a percentage of revenue) increased to 25.6% during the three months ended September 30, 2022, from 8.6% during the three months ended September 30, 2021.
Total operating expenses for the third quarter of 2022, were $4,976,000, an increase of $853,000, or 20.7% compared to $4,123,000 for the third quarter of 2021. This increase was primarily due to increases in expenses related to the commencement of the C-Guardians FDA study and share-based compensation expenses.
Net loss for the third quarter of 2022 totaled $4,529,000, or $0.58 per basic and diluted share, compared to a net loss of $4,071,000, or $0.53 per basic and diluted share, for the same period in 2021.
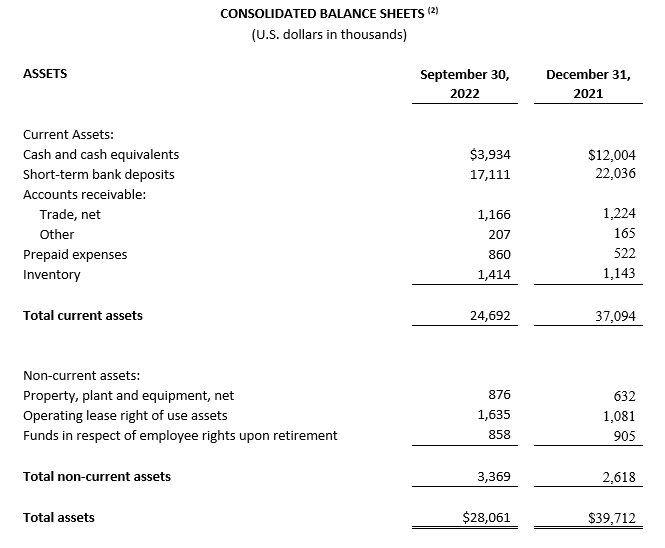
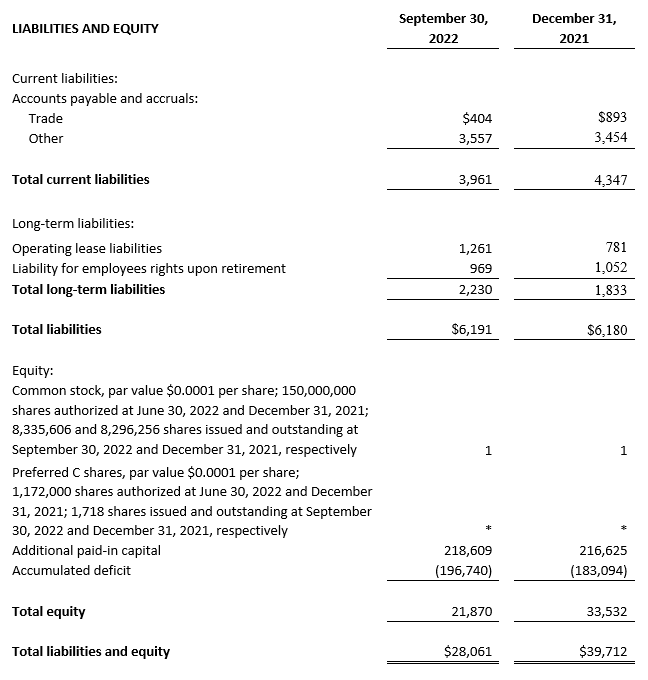
As of September 30, 2022, cash, cash equivalents and short-term bank deposits were $21.0 million compared to $34.0 million as of December 31, 2021.
Financial Results for the Nine Months ended September 30, 2022
For the nine months ended September 30, 2022, total revenue increased by $1,030,000, or 33.1%, to $4,145,000, from $3,115,000 during the nine months ended September 30, 2021. This increase was predominantly driven by a 35.7% increase in sales of CGuard EPS, to $4,097,000 during the nine months ended September 30, 2022 from $3,018,000 during the nine months ended September 30, 2021. This sales increase was mainly due to growth in existing and new markets and sales in the United States related to stents used in our C-Guardians FDA study as enrollment accelerated.
Gross profit for the nine months ended September 30, 2022 increased by $459,000, or 99.8%, to $919,000, compared to a gross profit of $460,000 for the nine months ended September 30, 2021. This increase in gross profit resulted from higher revenue, partially offset by a decrease of $141,000 in miscellaneous expenses. Gross margin (gross profits as a percentage of revenue) increased to 22.2% during the nine months ended September 30, 2022 from 14.8% during the nine months ended September 30, 2021.
Total operating expenses for the nine months ended September 30, 2022, were $14,696,000, an increase of $3,451,000, or 30.7% compared to $11,245,000 for the nine months ended September 30, 2021. This increase was primarily due to increases in expenses related to the commencement of the C-Guardians FDA study, share-based compensation, resumed activities in tradeshows and travel, directors’ and officers’ liability insurance and miscellaneous expenses.
Net loss for the nine months ended September 30, 2022 totaled $13,646,000, or $1.75 per basic and diluted share, compared to a net loss of $10,821,000, or $1.50 per basic and diluted share, for the nine months ended September 30, 2021.
Conference Call and Webcast Details
Management will host a conference call at 8:30AM ET today, November 8th, to review financial results and provide an update on corporate developments. Following management’s formal remarks, there will be a question-and-answer session.
Tuesday, November 8th at 8:30 a.m. ET
Domestic: 1-877-407-4018
International: 1-201-689-8471
Conference ID: 13733319
Webcast: Webcast Link – Click Here
About InspireMD, Inc.
InspireMD seeks to utilize its proprietary MicroNet™® technology to make its products the industry standard for carotid stenting by providing outstanding acute results and durable, stroke-free, long-term outcomes. InspireMD’s common stock is quoted on the Nasdaq under the ticker symbol NSPR.
For more information, please visit www.inspiremd.com.
Forward-looking Statements
This press release contains “forward-looking statements.” Such statements may be preceded by the words “intends,” “may,” “will,” “plans,” “expects,” “anticipates,” “projects,” “predicts,” “estimates,” “aims,” “believes,” “hopes,” “potential”, “scheduled” or similar words. Forward-looking statements are not guarantees of future performance, are based on certain assumptions and are subject to various known and unknown risks and uncertainties, many of which are beyond the company’s control, and cannot be predicted or quantified and consequently, actual results may differ materially from those expressed or implied by such forward-looking statements. Such risks and uncertainties include, without limitation, risks and uncertainties associated with (i) market acceptance of our existing and new products, (ii) negative clinical trial results or lengthy product delays in key markets, (iii) an inability to secure regulatory approvals for the sale of our products, (iv) intense competition in the medical device industry from much larger, multinational companies, (v) product liability claims, (vi) product malfunctions, (vii) our limited manufacturing capabilities and reliance on subcontractors for assistance, (viii) insufficient or inadequate reimbursement by governmental and other third party payers for our products, (ix) our efforts to successfully obtain and maintain intellectual property protection covering our products, which may not be successful, (x) legislative or regulatory reform of the healthcare system in both the U.S. and foreign jurisdictions, including the changing regulatory environment in Europe and the timing of the renewal of certificate to continue to sell CGuard under the new MDR rule structure, (xi) our reliance on single suppliers for certain product components, (xii) the fact that we will need to raise additional capital to meet our business requirements in the future and that such capital raising may be costly, dilutive or difficult to obtain and (xiii) the fact that we conduct business in multiple foreign jurisdictions, exposing us to foreign currency exchange rate fluctuations, logistical and communications challenges, burdens and costs of compliance with foreign laws and political and economic instability in each jurisdiction. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is set forth in the Company’s filings with the Securities and Exchange Commission (SEC), including the Company’s Annual Report on Form 10-K and its Quarterly Reports on Form 10-Q. Investors and security holders are urged to read these documents free of charge on the SEC’s web site at http://www.sec.gov. The Company assumes no obligation to publicly update or revise its forward-looking statements as a result of new information, future events or otherwise.
Investor Contacts:
Craig Shore
Chief Financial Officer
InspireMD, Inc.
888-776-6804
craigs@inspiremd.com
Chuck Padala, Managing Director
LifeSci Advisors
646-627-8390
chuck@lifesciadvisors.com
investor-relations@inspiremd.com
(1) All 2022 financial information is derived from the Company’s 2022 unaudited financial statements, as disclosed in the Company’s Quarterly Report on Form 10-Q, filed with the Securities and Exchange Commission; all 2021 financial information is derived from the Company’s 2021 unaudited financial statements, as disclosed in the Company’s Quarterly Report on Form 10-Q, filed with the Securities and Exchange Commission.
(2) All September 30, 2022 financial information is derived from the Company’s 2022 unaudited financial statements, as disclosed in the Company’s Quarterly Report on Form 10-Q, filed with the Securities and Exchange Commission. All December 31, 2021 financial information is derived from the Company’s 2021 audited financial statements as disclosed in the Company’s Annual Report on Form 10-K, for the twelve months ended December 31, 2021 filed with the Securities and Exchange Commission.
