Tel Aviv, Israel—June 2, 2017 – InspireMD, Inc. (NYSE MKT:NSPR) (NYSE MKT:NSPR.WS) (“InspireMD” or the “Company”), a leader in embolic prevention systems (EPS) / thrombus management technologies and neurovascular devices, today announced that Paul Stuka, who has served on the Company’s Board of Directors since 2011, has been appointed Chairman of the Board, replacing Sol Barer, Ph.D., who is stepping down from the Board due to conflicting professional commitments, but will serve as a special advisor to the Board.
Mr. Stuka currently serves as the managing member of Osiris Partners, LLC, an investment fund, since 2000. Prior to forming Osiris Partners, LLC, Mr. Stuka was a managing director of Longwood Partners, managing small cap institutional accounts. In 1995, Mr. Stuka joined State Street Research and Management as manager of its Market Neutral and Mid Cap Growth Funds. From 1986 to 1994, Mr. Stuka served as the general partner of Stuka Associates, where he managed a U.S. based investment partnership. Mr. Stuka began his career in 1980 as an analyst at Fidelity Management and Research. As an analyst, Mr. Stuka followed a wide array of industries including healthcare, energy, transportation, and lodging and gaming. Early in his career he became the assistant portfolio manager for three Fidelity Funds, including the Select Healthcare Fund which was recognized as the top performing fund in the United States for the five-year period ending December 31, 1985.
James Barry, Ph.D., Chief Executive Officer of InspireMD, commented, “Both the management team and the Board are excited with the appointment of Paul as our new Chairman of the Board. Paul not only brings over 35 years of capital markets experience, which will be extremely valuable as we enter the next phase of our growth, but he also knows our company well given his role as a Director since 2011. He has an impressive track record helping micro- and small-cap companies navigate the capital markets and maximize shareholder value.”
Mr. Stuka commented, “I am honored to become Chairman of InspireMD. Recent responses to our technology from key opinions leaders has been overwhelmingly positive and we look forward to capitalizing on this excitement as we rapidly grow our distribution network throughout the world. First and foremost, we are committed to maximizing value for shareholders by continued revenue growth, careful management of our expenses, and an eye on potential partnerships across our technology platform. I would also like the thank Sol Barer for his tremendous contributions as Chairman of the Board. His guidance and support over the years has been invaluable and we are grateful for his willingness to serve as a special advisor to the Board.”
Dr. Barer concluded, “I remain confident in the outlook for the Company. Although my current responsibilities preclude me from serving on the Board, I look forward to remaining an active advisor, supporter and major shareholder of the Company.”
About InspireMD, Inc.
InspireMD seeks to utilize its proprietary MicroNet™ technology to make its products the industry standard for embolic protection and to provide a superior solution to the key clinical issues of current stenting in patients with a high risk of distal embolization, no reflow and major adverse cardiac events.
InspireMD intends to pursue applications of this MicroNet™ technology in coronary, carotid (CGuard™), neurovascular, and peripheral artery procedures. InspireMD’s common stock is quoted on the NYSE MKT under the ticker symbol NSPR and certain warrants are quoted on the NYSE MKT under the ticker symbol NSPR.WS.
Forward-looking Statements
This press release contains “forward-looking statements.” Such statements may be preceded by the words “intends,” “may,” “will,” “plans,” “expects,” “anticipates,” “projects,” “predicts,” “estimates,” “aims,” “believes,” “hopes,” “potential” or similar words. Forward-looking statements are not guarantees of future performance, are based on certain assumptions and are subject to various known and unknown risks and uncertainties, many of which are beyond the Company’s control, and cannot be predicted or quantified and consequently, actual results may differ materially from those expressed or implied by such forward-looking statements. Such risks and uncertainties include, without limitation, risks and uncertainties associated with (i) market acceptance of our existing and new products, (ii) negative clinical trial results or lengthy product delays in key markets, (iii) an inability to secure regulatory approvals for the sale of our products, (iv) intense competition in the medical device industry from much larger, multinational companies, (v) product liability claims, (vi) product malfunctions, (vii) our limited manufacturing capabilities and reliance on subcontractors for assistance, (viii) insufficient or inadequate reimbursement by governmental and other third party payers for our products, (ix) our efforts to successfully obtain and maintain intellectual property protection covering our products, which may not be successful, (x) legislative or regulatory reform of the healthcare system in both the U.S. and foreign jurisdictions, (xi) our reliance on single suppliers for certain product components, (xii) the fact that we will need to raise additional capital to meet our business requirements in the future and that such capital raising may be costly, dilutive or difficult to obtain and (xiii) the fact that we conduct business in multiple foreign jurisdictions, exposing us to foreign currency exchange rate fluctuations, logistical and communications challenges, burdens and costs of compliance with foreign laws and political and economic instability in each jurisdiction. More detailed information about the Company and the risk factors that may affect the realization of forward looking statements is set forth in the Company’s filings with the Securities and Exchange Commission (SEC), including the Company’s Annual Report on Form 10-K and its Quarterly Reports on Form 10-Q. Investors and security holders are urged to read these documents free of charge on the SEC’s web site at http://www.sec.gov. The Company assumes no obligation to publicly update or revise its forward-looking statements as a result of new information, future events or otherwise.
Investor Contacts:
InspireMD, Inc.
Craig Shore
Chief Financial Officer
Phone: 1-888-776-6804 FREE
Email: craigs@inspiremd.com
Crescendo Communications, LLC
David Waldman
Phone: (212) 671-1021
Email: NSPR@crescendo-ir.com

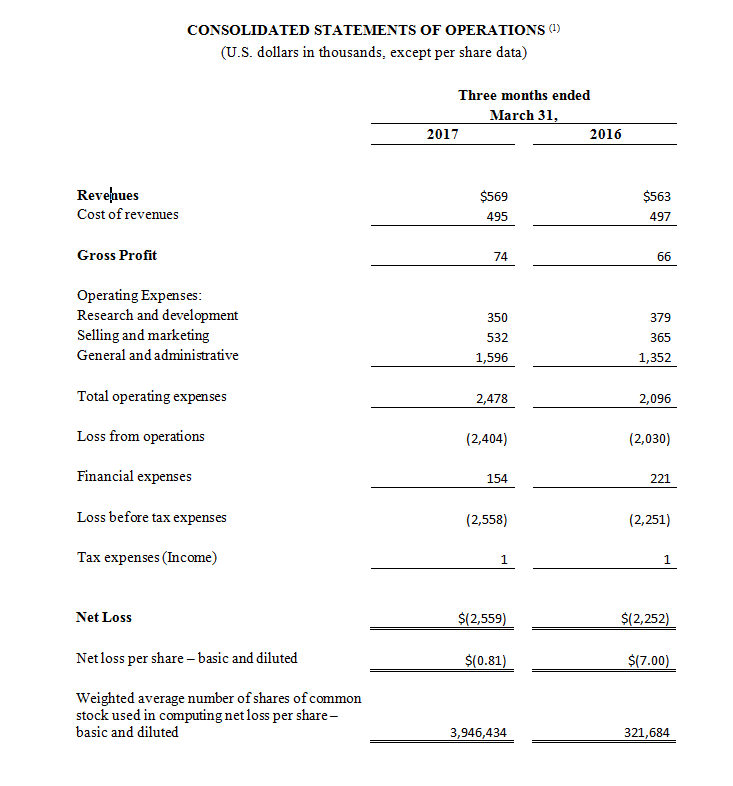
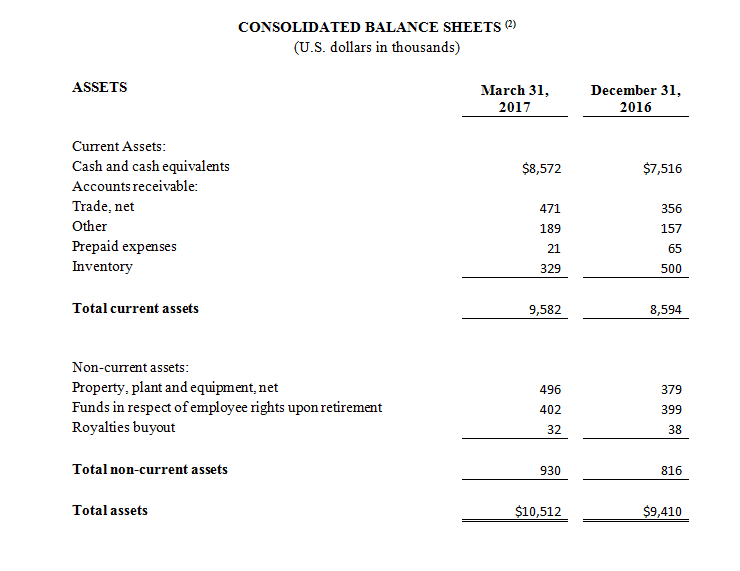
 (1) All 2017 financial information is derived from the Company’s 2017 unaudited financial statements, as disclosed in the Company’s Quarterly Report on Form 10-Q, filed with the Securities and Exchange Commission, all 2016 financial information is derived from the Company’s 2016 unaudited financial statements, as disclosed in the Company’s Quarterly Report on Form 10-Q, filed with the Securities and Exchange Commission.
(1) All 2017 financial information is derived from the Company’s 2017 unaudited financial statements, as disclosed in the Company’s Quarterly Report on Form 10-Q, filed with the Securities and Exchange Commission, all 2016 financial information is derived from the Company’s 2016 unaudited financial statements, as disclosed in the Company’s Quarterly Report on Form 10-Q, filed with the Securities and Exchange Commission.