100% procedural success rate in a 30 patient study using the new CGuardTM Embolic Prevention System with no procedural related complications and zero strokes at 6-month follow up
Companion Editorial Highlights Need for More Effective Management of Carotid Artery Stenosis for Stroke Prevention
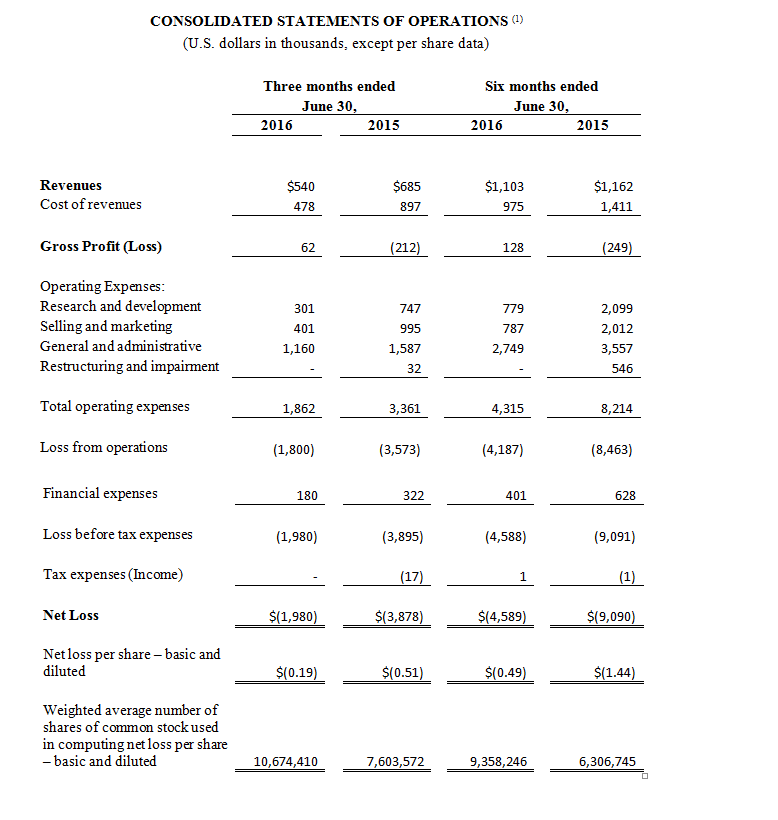
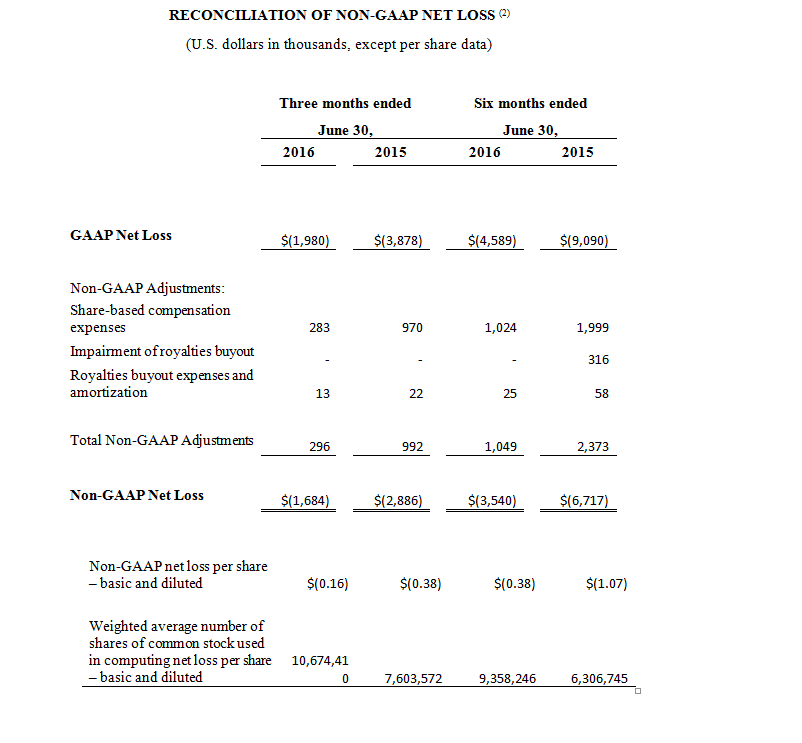
BOSTON, MA – October 17, 2016 – InspireMD, Inc. (NYSE MKT: NSPR, NSPR.WS) (“InspireMD” or the “Company”), a leader in embolic prevention systems (EPS), neurovascular devices and thrombus management technologies, today announced the online publication of positive clinical data in a new independent study performed by Prof. Christian Wissgott, M.D., entitled “Clinical Results and Mechanical Properties of the Carotid CGUARDTM Double-Layered Embolic Prevention Stent,” which evaluated the Company’s CGuard™ EPS in patients with symptomatic or high-grade asymptomatic internal carotid artery (ICA) stenosis. The study, published in the Journal of Endovascular Therapy online-ahead-of-print, is available digitally on the journal’s OnlineFirst website section. The OnlineFirst section also includes an accompanying editorial written by Prof. Piotr Musialek, M.D., entitled “Carotid Artery Revascularization for Stroke Prevention: A New Era,” which underscores the use of CGuardTM EPS as a novel approach to potentially reducing the risk of stroke by avoiding the risks associated with conventional carotid stenting.
Prof. Christian Wissgott, M.D., Assistant Director at Westkustenklinikum, Heide, Germany, independently evaluated CGuard™ properties against other carotid stents and conducted a study in 30 consecutive patients with Internal Carotid Artery Stenosis (ICA) disease. The average stenosis of the treated arteries was 84% with a mean lesion length of 17 mm. The majority of the patients (83%) had symptomatic disease. Patients were followed for six months post-procedure and were assessed using a number of variables, including stroke, change in modified Rankin Scale (mRS), CGuardTM EPS patency, and new ipsilateral lesions as measured by DW-MRI. Key findings from the six month study included:
- 100% success in implanting the CGuardTM EPS;
- No peri- or post-procedural complications;
- No deaths, major adverse events, minor or major strokes, or new neurologic symptoms during the six months following the procedure;
- Modified Rankin Scale improved for the symptomatic patients from 1.56 prior to the procedure to 0 afterwards;
- All vessels treated with the CGuardTM system remained patent (open) at six months; and
- DW-MRI performed in 19 of 30 patients found no new ipsilateral lesions after 30 days and after six months compared with the baseline DW-MRI studies.
Additionally, based on engineering evaluations, the study concluded that the CGuardTM EPS provides a high radial force and strong support in stenotic lesions, and its structure, in contrast to some other stents, adapted well to changes in vessel diameter and direction. The MicroNet™ mesh of the CGuardTM did not cause any changes to specific mechanical parameters of the underlying stent.
“The novel construction of the CGuardTM EPS prevented post-procedural embolic events in this series of patients undergoing routine carotid artery stenting,” said Prof. Wissgott. “The CGuardTM EPS is easy and safe to implant because it more readily adapts to the shape and diameter of the vessel wall versus other carotid artery stents. Importantly, none of the patients in this series experienced any complications or strokes as a result of the procedure or in the following six months. Consequently, I believe that the CGuardTM EPS is an important new treatment option for both symptomatic and asymptomatic carotid artery stenosis patients.”
Key points highlighted in the companion editorial from Piotr Musialek, M.D., DPhil, FESC, Prof. in the Jagiellonian University Department of Cardiac & Vascular Diseases include:
- 85% of strokes occur without warning signs, raising questions about the validity of “watchful waiting” in patients with asymptomatic carotid artery stenosis;
- Novel stent technology ensures viability of endovascular approaches to carotid revascularization in both primary and secondary stroke prevention;
- Consistent clinical evidence is accumulating that demonstrates that mesh covered stents, with InspireMD’s CGuardTM EPS in particular, prevent post-procedural embolic events; and
- Data in Wissgott et al is consistent with the positive outcomes reported in all other clinical trials of CGuard™.
“Given the risks associated with prior carotid stent options to date, many physicians prefer to treat their asymptomatic patients with medication-only rather than medication plus interventional plaque pacification,” said Prof. Musialek. He continued, “The growing body of data for the CGuardTM EPS demonstrates that this novel system may have a much lower risk of peri- or post-procedural complications and embolic events. Prof. Wissgott’s study and the results of the CARENET and PARADIGM 101 studies demonstrate that CGuardTM EPS may help to reduce the risk of stroke for asymptomatic patients and provide them with improved long-term cerebrovascular outcomes. There is little to no doubt today that a new carotid revascularization era has arrived.”
About CGuard™ EPS
The CGuard™ EPS is designed to prevent peri-procedural and late embolization by trapping potential emboli against the arterial wall while maintaining excellent perfusion to the external carotid artery and branch vessels.
MicroNet™ is a bio-stable mesh woven from a single strand of 20 micron Polyethylene Terephthalate (PET).
CGuard™ EPS is CE Marked and not approved for sale in the U.S. by the U.S. Food and Drug Administration at this time.
Carotid stenosis is a narrowing of the carotid arteries, the major arteries that supply blood and oxygen to the brain. This narrowing results from a buildup of plaque inside the blood vessel and reduces blood flow to the brain. The presence of plaque in the blood vessel can also cause the development of blood clots, which may also reduce blood flow to the brain. In some cases, plaque may rupture or dislodge from the vessel wall and block smaller downstream arteries. Patients with carotid stenosis have an increased risk of stroke as a result of cerebral embolism and decreased blood flow to the brain.
Patients with symptomatic carotid stenosis are typically treated by placement of a stent inside the blood vessel in order to re-open the carotid artery and improve blood flow to the brain. InspireMD’s CGuardTM EPS uses the company’s patented MicroNet™ technology to provide the revascularization benefits of a stent with a mesh “safety net” that secures the plaque against the blood vessel’s arterial wall and thereby prevents plaque and other debris from flowing through the stent’s scaffold.
About InspireMD, Inc.
InspireMD seeks to utilize its proprietary MicroNet™TM technology to make its products the industry standard for embolic protection and to provide a superior solution to the key clinical issues of current stenting in patients with a high risk of distal embolization, no reflow and major adverse cardiac events.
InspireMD intends to pursue applications of this MicroNet™ technology in coronary, carotid (CGuardTM), neurovascular, and peripheral artery procedures. InspireMD’s common stock is quoted on the NYSE MKT under the ticker symbol NSPR and certain warrants are quoted on the NYSE MKT under the ticker symbol NSPR.WS.
Forward-looking Statements
This press release contains “forward-looking statements.” Such statements may be preceded by the words “intends,” “may,” “will,” “plans,” “expects,” “anticipates,” “projects,” “predicts,” “estimates,” “aims,” “believes,” “hopes,” “potential” or similar words. Forward-looking statements are not guarantees of future performance, are based on certain assumptions and are subject to various known and unknown risks and uncertainties, many of which are beyond the Company’s control, and cannot be predicted or quantified and consequently, actual results may differ materially from those expressed or implied by such forward-looking statements. Such risks and uncertainties include, without limitation, risks and uncertainties associated with (i) market acceptance of our existing and new products, (ii) negative clinical trial results or lengthy product delays in key markets, (iii) an inability to secure regulatory approvals for the sale of our products, (iv) intense competition in the medical device industry from much larger, multinational companies, (v) product liability claims, (vi) product malfunctions, (vii) our limited manufacturing capabilities and reliance on subcontractors for assistance, (viii) insufficient or inadequate reimbursement by governmental and other third party payers for our products, (ix) our efforts to successfully obtain and maintain intellectual property protection covering our products, which may not be successful, (x) legislative or regulatory reform of the healthcare system in both the U.S. and foreign jurisdictions, (xi) our reliance on single suppliers for certain product components, (xii) the fact that we will need to raise additional capital to meet our business requirements in the future and that such capital raising may be costly, dilutive or difficult to obtain and (xiii) the fact that we conduct business in multiple foreign jurisdictions, exposing us to foreign currency exchange rate fluctuations, logistical and communications challenges, burdens and costs of compliance with foreign laws and political and economic instability in each jurisdiction. More detailed information about the Company and the risk factors that may affect the realization of forward looking statements is set forth in the Company’s filings with the Securities and Exchange Commission (SEC), including the Company’s Annual Report on Form 10-K and its Quarterly Reports on Form 10-Q. Investors and security holders are urged to read these documents free of charge on the SEC’s web site at http://www.sec.gov. The Company assumes no obligation to publicly update or revise its forward-looking statements as a result of new information, future events or otherwise.
Investor Contacts:
InspireMD, Inc.
Craig Shore
Chief Financial Officer
Phone: 1-888-776-6804 FREE
Email: craigs@inspiremd.com
Lazar Partners
David Carey
Investor Relations
(212) 867-1768
Email: dcarey@lazarpartners.com